The transition deadline for new requirements on Dihydroxyacetone in cosmetic products is approaching. Hair dyes and self-tanning products not in compliance with the new restrictions must not be available on EU markets from April 22, 2022.
Published by the European Commission (EC), Regulation (EU) 2021/1099 amended requirements in the Cosmetics Regulation (EC) 1223/2009 relating to the use of Tetrahydropyranyloxy Phenol (CAS No. 53936-56-4) and Dihydroxyacetone (CAS No. 96-26-4) in cosmetic products. The new restrictions came into force in July 2021.
While the prohibition on the use of Tetrahydropyranyloxy Phenol came into effect on July 26, 2021, the Commission allowed for a transition period in relation to Dihydroxyacetone to assist the industry. The first transition period deadline has already elapsed and, since January 26, 2022, new cosmetic products placing onto the EU market must comply with the regulation’s requirements for Dihydroxyacetone.
The second transition deadline is April 22, 2022. From this date, cosmetic products placed onto the EU market prior to the enforcement date, and which do not comply with the new requirements for Dihydroxyacetone, must be removed from sale and cannot be made available on the market.
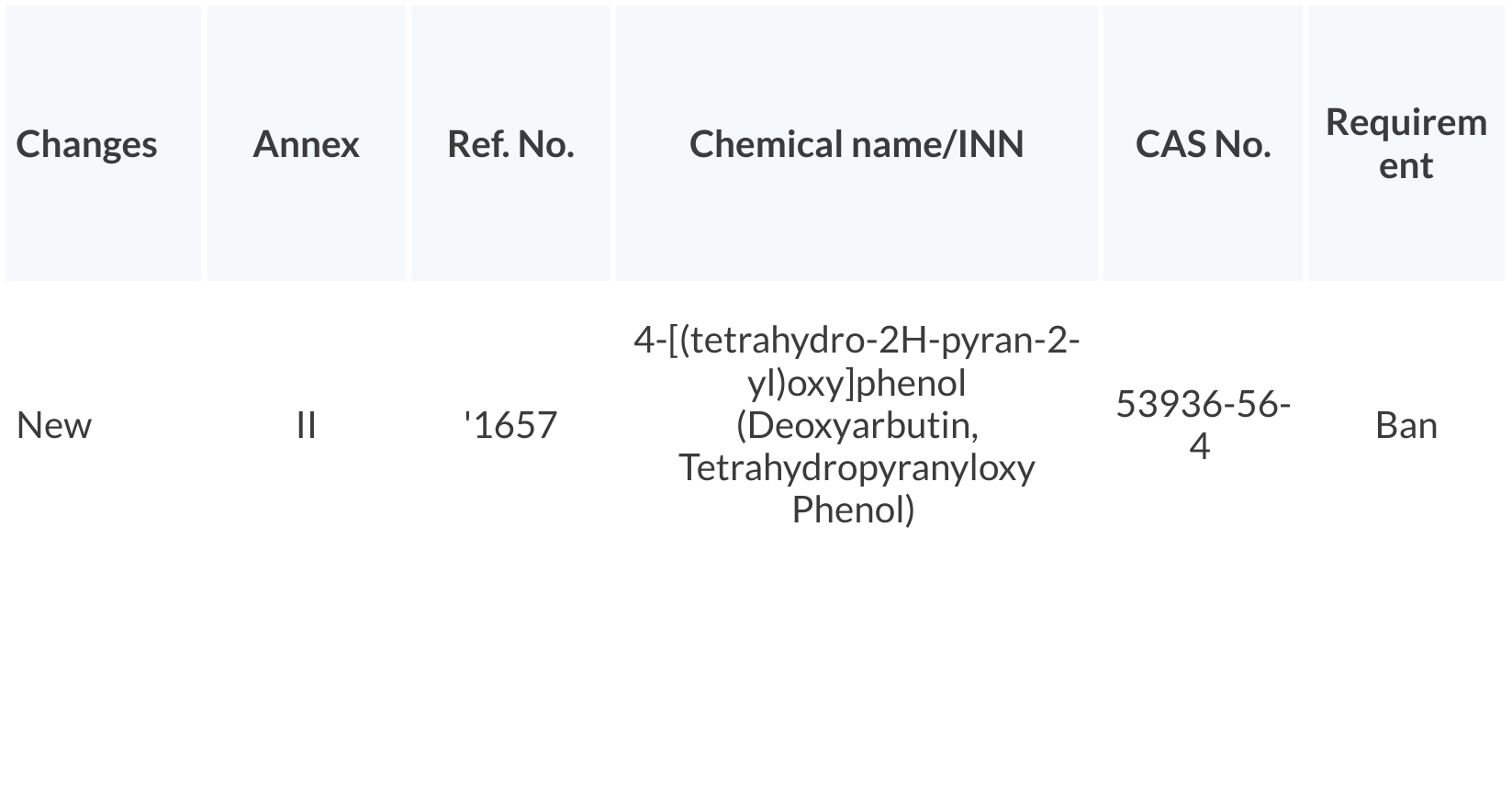
Prohibition on the use of Tetrahydropyranyloxy Phenol in cosmetic products – enforcement date July 26, 2021.
Click on the table to enlarge.
New restrictions on the use of Dihydroxyacetone:
- Now – products not complying with the restrictions shall not be placed on the EU market
- From April 22, 2022 – products not complying with the restrictions shall not be made available on the EU market
Click on the table to enlarge.
NEXT STEP
The responsible person shall withdraw the cosmetic products not complying to the new requirement of Dihydroxyacetone as soon as possible.
Queenie Tse
Cosmetics & Hygiene – Technical Services Manager



.png?width=570&name=Deadline-Approaches-for-Compliance-with-EU-Regulation-2-table%20(1).png)