Italy
Italy’s Ministry of Agricultural, Food and Forestry Policies has issued a ministerial decree which extends the national provisions in force beyond April 1, 2020, the date of entry into application of European regulation 2018/775 on the provision of food information to consumers, with regard to rules for indicating the country of origin, or place of provenance, of the primary ingredient of a food.
More specifically, this ministerial decree has been issued in order to defend the transparency of the food label information for consumers in relation to pasta, rice, and processed tomato products and to support those food companies now facing problems in complying with EU regulation 2018/775, due to the temporary closure of packaging companies across Europe during the COVID-19 pandemic.
It is clear that this ministerial decree also intended to protect three key sectors in the Italian agri-food system which are now, more than ever, under the pressure of continuous demand for quality food at affordable prices, while facing the difficulties of an economy in contraction.
European Union (EU)
The EU Commission is implementing regulation (EU) 2020/466 that defines temporary derogations and requirements of official control, that support the official control system during the COVID-19 state of emergency. The implementation was published on March 30, 2020 in OJ-EU, and came into force on the April 1,2020.
The derogations of official control requirements pursuant to Reg. N. 2017/625, include conditions involving external personnel for carrying out official control activities, the acceptability of electronic forms of official certificates, exceptional recourse to laboratories other than the official ones, and the use of remote means of communication.
A draft amendment to Regulation (EC) No 1925/2006 on botanical species (including Aloe Vera) containing hydroxyanthracene derivatives (HADs) has been issued, adding them to the list of restricted or prohibited substances.
After the publication of the EFSA 2013 opinion, different Member States raised concerns on the authorization of hydroxyanthracene derivatives-based products in food, and the European Commission asked EFSA for a more in-depth investigation. Before and after the EFSA 2013 opinion on HADs, different international bodies and organizations performed their own evaluations based on the available literature and new data regarding the efficacy and safety for use of these substances (IARC, EMA, BfR, and others). In 2018, EFSA published a scientific opinion on HADs, in which these substances showed genotoxicity in vitro and in vivo studies, concluding that a safe daily intake could not be determined.
Consequently, the Authority has decided that aloe-emodin, emodin, danthron, and hydroxyanthracene derivative-containing aloe extracts should be included in Annex III, Part A of Regulation (EC) No 1925/2006, which includes the substances whose use in foods is prohibited, restricted or under Union scrutiny.
United Kingdom (UK)
The UK’s Food Standards Agency (FSA), together with the Department for Environment, Food and Rural Affairs (Defra) has published a guidance document to support food businesses during the coronavirus disease pandemic.
Although the transmission of COVID-19 by food is not supported by any evidence, food-related businesses, particularly in this critical moment, need to comply with the general good hygiene practices, the control of employee sickness and the physical distancing to further prevent the spread of the disease. Read more on Digicomply
~
A rapid increase in the drinking of raw milk in England and Wales in recent years has been followed by an increase in outbreaks of illnesses linked to its consumption. The UK’s Food Standards Agency (FSA) has published guidance document on raw milk for drinking to tighten control of this sector, while preserving consumer choice and the related businesses.
The lack of pasteurization, which kills key pathogens and prolongs the shelf-life of these products, is a critical aspect that might lead to the rapid spoilage of raw milk. From now on, the raw milk (for drinking) producers will need to follow the specific safety measures highlighted in this guidance document to ensure that their products satisfy the minimum safety requirements. FSA Dairy Hygiene Inspectors visit farms on a six-month basis to check the implementation of these safety measures.
As general advice, the FSA does not recommend the consumption of raw milk for pregnant women, infants and toddlers, elderly and immunocompromised persons. Read more on Digicomply
Central America
Mexico’s new regulation, on food additives, was expected to come into force in June 2020, as per the annex published in COMIECO Resolution 419-2019. This process was interrupted by the judge of the Seventh District Court for Administrative Matters in February this year, in the form of the provisional suspension of Official Mexican Standard 051.
The new front of packaging labeling of food and beverages is focused on addressing obesity. Previous reform of the General Health Law has resulted in the use of black octagon labels on prepacked foods with warnings about excess sugars, fats, calories, and sodium. This caused confrontation between the private sector and consumer advocacy groups, which consequently raised concerns about the transparency of this process. Read more on Digicomply
United States of America (USA)
On March 17, 2020, The U.S. Food and Drug Administration (FDA) Issued a Temporary Policy for Food Safety Modernization Act (FSMA) supplier verification onsite audit requirements, that assures the functionality of the food supply chain during the coronavirus (COVID-19) public health emergency. Under the new circumstances, the agency will not enforce regular onsite audit checks, but instead will temporarily allow other appropriate verification methods, such as sampling or a food safety records review that will provide enough evidence of safety. Read more on Digicomply
~
Another temporary flexibility granted by the U.S. FDA, during the COVID-19 pandemic, refers to the implementation of the Nutrition Facts label for restaurants and food manufacturers wishing to resell packaged food to consumers, or businesses for sale to consumers.
It is granted only in the cases in which the packaged food does not contain any nutrition claims while other required information is available, like the identity of food, an ingredient statement, the name and location of business of the food manufacturer, packer, or distributor, the net quantity of contents, and standard information on any allergens present.
The new labeling guidelines on Nutrition Facts statements for packed foods was finalized at the beginning of March 2020. The new design of the Nutrition Facts panel is based on the current scientific information and strongly supported by the Education Campaign of U.S. FDA. It is designed to increase awareness of healthy diet and food choices. An important and novel approach is the inclusion of information that makes the connection between diet and chronic disease.
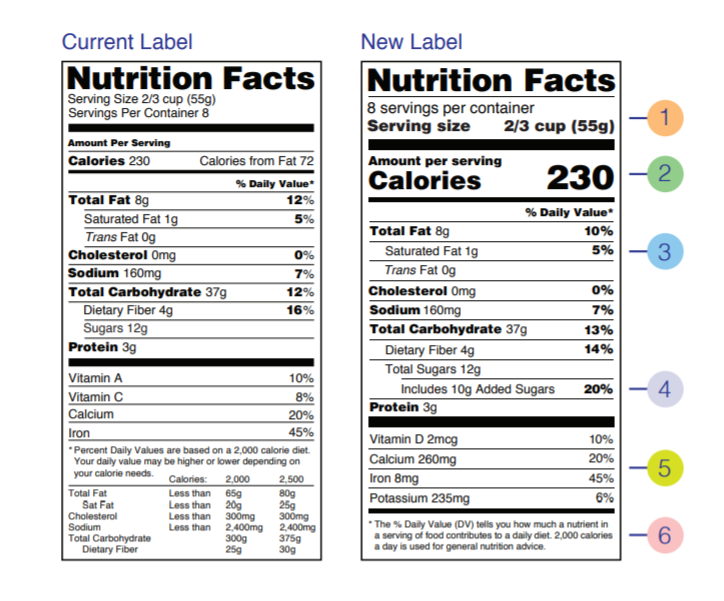
The type size was increased on some items that the FDA wants to be noticed (such as serving and package) size, the listing for calories from fat removed, the added sugar listing made mandatory, the required nutrients list updated, and the footnote at the bottom of the label to better explain percentage daily value updated too.
~
A Food Safety Attorney has petitioned the USDA FSIS to ban 31 antibiotic-resistant Salmonella strains from meat and poultry, and to label several Salmonella outbreak serotypes as adulterants. Read more on Digicomply
The USDA has extended the comment period by 60 days for the petition to classify 31 strains of Salmonella as adulterants in meat and poultry products. Read more on Digicomply
Singapore
Respected Singapore authorities are seeking feedback and comments from stakeholders on amendments to the Food Regulations, proposed in June 2019, to ban Partially Hydrogenated Oils (PHOs) in all foods sold, imported and those locally manufactured as well as pre-packaged in the country.
The amendments will come into force in June 2021 and will replace the existing 2% limit on trans fat content in fats and oils sold. Read more on Digicomply
South Korea
Korea’s Ministry of Food and Drug Safety performed analysis on 66 samples from 14 types of seafood to evaluate Microplastic residues and concluded that they do not represent a risk to health for those consuming aquatic products.
Brazil
The Brazilian Health Surveillance Agency published a FAQ on food microbiological standards and regulations (First Edition) on March 16, 2020. The regulations were revised in December 2019 and will take effect in December 2020.
Hong Kong
The Center for Food Safety (CFS) introduced new measures for Online Application for Import License and Import Permit Service and Food Import Control. This measure introduced new measures to streamline import permit requirements, blue paper pattern and application forms. It also updated requirements for the submission of health certificates and export declarations, and introduced a new "CFS Food Code" that will improve food traceability. Submitting an application through the dedicated website will possible at any time, while the License Issuance Office will extend its daily operating hours.
Russia
Due to the global epidemiological situation, the Russian Federal Service for Veterinary and Phytosanitary Surveillance has simplified the import procedure for cargo clearance for plant and animal products. By the notice published on March 20, 2020, registration will be carried out according to the necessary documentations of this body, with the competent services of other countries. Read more on Digicomply
~
In the same period, during quarantine phytosanitary control, between March 16 and March 22, 2020, 13 quarantined objects were identified for the Eurasian Economic Union, out of 112 cases of products imported into the territory of the Russian Federation. Read more on Digicomply
~
According to Rosstat, the volume of production of chocolate and cocoa-containing food products in Russia increased by 17.6%, in 2019. Read more on Digicomply
Australia and New Zealand
Pregnancy warning labels on alcoholic beverages was one of the topics at the Australia and New Zealand Ministerial Forum on Food Regulation meeting held on March 20, 2020. A voluntary pregnancy warning labeling scheme was implemented by the alcohol industry in 2011, which is now being reviewed through Proposal P1050 to be implemented as a standard. Ministers requested that this parameter review be undertaken quickly, due to the cost burden on industry, and asked to notify the Forum within three months. The next meeting of the Forum is scheduled for May 2020.
Like this summary? You can create your very own with Digicomply Compliance Watch, register for free




.webp?width=1644&height=1254&name=Food%20Safety%20Dashboard%201%20(1).webp)
