Ⅰ. Background to the revision of the standard
The current standard for nutrition labels for prepackaged food in China is GB28050-2011 National Food Safety Standard General Principles for Nutrition Labels for Prepackaged Food (the standard). To adapt to changing needs for food nutrition labels, in 2016, the Chinese government set up a project to organize, unify and revise the standard.
On July 19, 2021, the second session of The Professional Committee of the National Food Safety Standards Review Committee met to review and pass the new GB 28050-20×× National Food Safety Standard General Principles for Nutrition Labels for Prepackaged Food (draft) (hereinafter referred to as the draft).This is the third round of feedback, and relevant personnel were required to provide their input before November 21, 2021. The final standard has not yet been officially released.
To ensure that food industry practitioners have a clear understanding of the changes and future development trends for food nutrition labels in China, we have collated the revisions and differences between the current standard and new draft, and explain them here.
Ⅱ. The main revisions in the new draft standard are
- A revised scope of application
- An increase in the mandatory labeling of nutrients
- A definition added for energy and some nutrients
- Warnings have been added
- Optional labeling items have been modified and some supplementary information added
- The scope of the nutrition label exemption has been modified
- The allowable error range for some nutrient content has been modified
- Some Nutrient Reference Values (NRV) have been modified
- The nutrition label format has been revised
- Some standard terms for nutritional claims and functional claims for nutrients have been modified
- An appendix – Appendix E – has been added on recommended serving size reference values for prepackaged foods
Ⅲ. Details of and explanations for these changes
1. A revised scope of application
The scope of application is explicitly extended to include nutrition labels on packaged foods, and stored and transported packages that are not provided directly to consumers. This relates mainly to nutrition labels on some packages of B2B products. If nutritional information is included on those packages the draft standard should also be followed. This clarification will end confusion around the use of nutritional information on such packaging.
2. Requirements for imported pre-packaged foods have been clarified
Compared with the previous draft circulated for comment, the new draft clearly stipulates that the nutrition label content of imported pre-packaged food should comply with the provisions of this standard.
3. Change the label content from "1+4" to "1+6"
The “Draft” adds two mandatory labeling items: saturated fat (acid) and sugar.
Therefore, mandatory labeling contents will be revised from 1+4 (energy + protein, fat, carbohydrate, sodium) to 1+6 (energy + protein, fat, saturated fat (or saturated fatty acid), carbohydrate, sugar, sodium).
4. The scope of sugar has been clarified
The new draft refers specifically to the definition of sugar in the Codex, the United States and the European Union regulations, along with the basic concept of sugar in the international community. It states that the sugar listed on a nutrition label should be the sum of monosaccharides and disaccharides (excluding sugar alcohols).
5. Implement the concept of Great Health
Warning words have been added. Pre-packaged food should be clearly marked: "Children and adolescents should choose foods high in salt, fat and sugar in moderation". The aim is also, to promote a healthy lifestyle for all consumers, and to advocate the "Three Reduce" policy of dietary action, namely, reducing salt, oil and sugar.
6. Serving size reference values for prepackaged foods
When labeling the energy and nutrient content of a pre-packaged food by portion, the weight or volume (in edible parts) of each portion of the food may be determined by reference to the 18 pre-packaged food categories recommended in Appendix E. The purpose is to gradually guide enterprises and the market to standardize the usage of the expression of serving size.
7. Add claims for Energy, Sodium and Fat
The nutrition label can use graphics, text and other ways to supplement nutritional information on the front of a package.
For example, the rules allow the use of Oil and Salt familiar to consumers instead of Fat and Sodium, and the use of Calories instead of Kilojoules in energy description. Pagoda graphics and core recommendations in The Dietary Guidelines for Chinese Residents can be used to positively promote a reasonable diet and/or reduce the intake of salt, oil and sugar. The new draft enriches the choice and diversity of identification options.
8. The labeling and expression of nutritional ingredients have been revised and supplemented with the main changes which include:
-
When nutrients other than mandatory nutrients are marked, appropriate steps shall be taken to make the mandatory content mark more eye-catching. This could include increasing the font size, changing the font (bold, black, italic), changing the color (text or background color), etc.
-
The draft defines the way to obtain the marked value, using either the current national standard method, or a calculation based on the composition of raw materials using The Chinese Food Composition Table or other reliable data
-
The draft adds that the "0" limit value should meet the requirement of the "0" limit value per 100g or 100ml, when labeling the content of nutrients per portion. When labeled "0", the nutrient can also be labeled as "0.0" or "0.00" according to the modified interval of the nutrient
-
The draft adds details on the expression of units, rounding off and "0" thresholds for n-3 polyunsaturated fat (acid), α-linolenic acid, EPA and DHA. See Table 1 for details
-
Revision of the interval and "0" threshold requirements for vitamin A, vitamin E, vitamin B12, niacin (niacinamide) and zinc
Table 1, Names and Sequences, Units, Rounding Off and "0" thresholds of Energy and Nutrients
|
Energy and Nutrients |
Units |
Rounding Off |
"0" threshold value (per 100 g or 100 ml) |
|
Energy |
kJ |
1 |
≤17 kJ |
|
Protein |
g |
0.1 |
≤ 0.5 g |
|
Fat |
g |
0.1 |
≤ 0.5 g |
|
Saturated fat (or Saturated fatty acids) |
g |
0.1 |
≤ 0.1g |
|
Trans fatty acid |
g |
0.1 |
≤ 0.3g |
|
Mono-unsaturated fats (or mono-unsaturated fatty acids) |
g |
0.1 |
≤ 0.1g |
|
Poly-unsaturated fats (or Poly-unsaturated fatty acids) |
g |
0.1 |
≤ 0.1g |
|
n-3 poly-unsaturated fatty acids |
mg |
1 |
≤ 20 mg |
|
α- linolenic acid |
mg |
1 |
≤ 5 mg |
|
Eicosapentaenoic acid (EPA) |
mg |
1 |
≤ 5 mg |
|
Docosahexaenoic acid (DHA) |
mg |
1 |
≤ 5 mg |
9. Revision of tolerable error thresholds for some nutrient content
-
The tolerance threshold for vitamin A and vitamin D has been revised to be the same as that of other vitamins. Since it is not easy for the background content of vitamins A and D in food to exceed the threshold, and there is an upper limit for the fortification of vitamins A and D in GB14880, the original upper limit value is cancelled in this draft. This is now consistent with other vitamins, and the lower limit is specified
-
During the shelf life of a product, the rate of tolerable error between the announced values, and the actual measured or calculated values, of energy and nutrients in the food should comply with Table 2.
Table 2, Allowable error ranges for Energy and Nutrient content
|
Energy and Nutrients |
Tolerable Error |
|
Protein, Poly-unsaturated |
≥ 80% |
|
Energy in foods as well as |
≤ 120% |
10. Modification of the scope of the nutrition label exemption
-
The following expression of nutrition label exemption has been revised: Fresh food, such as livestock and poultry meat, aquatic products, vegetables and fruits, eggs, etc., Food with a maximum surface area of pre-packaged food packaging, or a packaging container ≤ 40cm2, and Daily consumption ≤10g(ml), of pre-packaged food or single raw material condiments.
-
The following expression of nutrition label exemption has been added: Dry products with single raw materials without adding other ingredients after simple physical treatment, such as cereals, dried fungi and algae fruits and vegetables.
-
Exemptions have been removed for Ready-made, ready-sold food and metered distribution of pre-packaged food.
11. The Nutrient Reference Value (NRV) was revised and the NRV for cholesterol deleted
-
The NRV for cholesterol have been deleted, and the NRV for vitamin D, Biotin, Choline, Zinc, Iodine, Selenium and Copper have been revised. The revised NRV contains energy and 31 nutrients
-
The population corresponding to the NRV value: NRVs are suitable for pre-packaged food nutrition labels for food products to be consumed by people aged 37 months and above
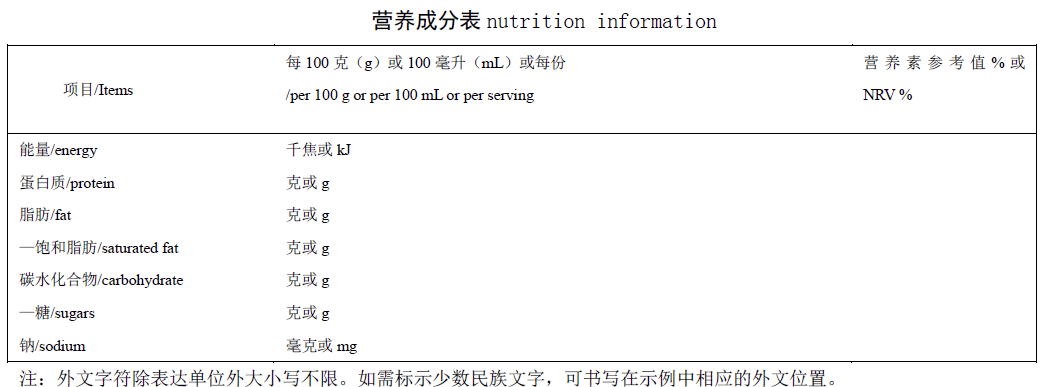
12. The recommended formats for nutrition labels has been increased from 6 to 8
Specific text descriptions for each format of nutrition label have been added to the new draft. It is easy for food suppliers to understand and apply. It has also been clearly expressed that the label format can be appropriately modified, providing that it does not mislead consumers.
Example: Multilingual format
13. Requirements for energy and nutrient content claims and comparison claims have been revised. The main changes:
-
States that energy claims, such as NO CALORIES, will be the same as a claim of NO ENERGY
-
Add requirements and claims for n-3 poly-unsaturated fatty acid
-
Specify the claims for dietary fiber: Source of soluble dietary fiber (or monomer), Contains soluble dietary fiber (or monomer), and High or rich in soluble dietary fiber (monomer)
-
Remove the claim of Low protein, and the restriction of No saturated fat claim
-
Revise the restriction condition of "degreasing" to Other dairy products should meet the corresponding national food safety standards
-
Revise the restriction of the Low saturated fat to Saturated fat energy ratio ≤10%
-
Amend the item Carbohydrate (sugar) to be separate entries for Sugar and Lactose, the corresponding content requirements and restrictive conditions remain unchanged
The revised comparison claims that Reference Food means: the measured data of food of the same kind or the same category or the same quality grade of the same enterprise; or from the same food data in The Chinese Food Composition Table.
More details are referenced in appendix C of the draft, which specifies the requirements, conditions and synonyms for energy and nutrient content claims of pre-packaged foods.
14. Adjustments to the standard wording for functional claims of multiple nutrients in Appendix D
-
The number of functional claims for nutrients have been increased, from 24 to 28
-
Functional claims have been added for α -linolenic acid, sugar, vitamin K, biotin, choline, phosphorus, potassium, selenium. In accordance with the Healthy China Concept, the standard language of functional claims, which requires adding warnings about the possible health effects of a long-term intake of high fat, sugar and salt shall be included. Also added is a statement that vitamin C helps maintain the normal physiological function of the immune system
-
The cholesterol function claim has been removed
15. Addition of Appendix E on recommended serving size reference values for prepackaged foods (18 categories recommended):
‘Serving Size Reference’ refers to the recommended weight or volume (counted as the edible part) per serving of a food item, when the nutrition label lists the nutritional facts per portion, or serving.
Table E.1 Recommended serving size for Prepackaged foods (g/ml) (count as edible part)
|
Cat. |
Serving Size |
|
Soy sauce, sauce, pickle, primer |
10g (or) ml |
|
Cooked dried meat products (meat floss, dried meat, preserved meat, etc.) |
10g |
|
Dried fruit |
10g |
|
Nuts |
10g |
|
Western style decorative cakes (pies, cakes) |
30g |
|
Meat filling products (sausage, western ham, etc.), ham products, fermented ham products, bacon products |
30g |
|
Milk powder (whole milk powder, skimmed milk powder, partially skimmed milk powder, modified milk powder) |
30g |
|
Instant cereal powder, meal substitute powder |
30g |
|
Biscuits, cereal bars |
30g |
|
Puffed food, potato chips, Guoba, dried steamed bread |
40g |
|
Bread and fermented flour products (steamed bread, rolls) |
50g |
|
Eggs (eggs, preserved eggs, salted eggs, marinated eggs, bad eggs, etc.) |
50g |
|
Ice cream |
50g(or)mL |
|
Instant noodles |
100g |
|
Fermented milk |
120g(or)mL |
|
Liquid milk (pasteurized milk, modified milk, sterilized milk) |
200mL |
|
Beverages |
200mL |
|
Soybean Milk |
200mL |
The draft clarifies the requirements for nutrition labeling and promotes the Three Reduce healthy lifestyle by adjusting the labeling of nutrition ingredients to facilitate the implementation of the Healthy China Action (2019-2030). At present, the draft is still in the consultation stage, and the specific implementation is subject to the standard final release by the Chinese government.
SGS is committed to keeping you informed of regulation news and developments. Leveraging our global network of laboratories and food experts, SGS provides a comprehensive range of food safety and quality solutions, including analytical tests, audits, certifications, inspections, and technical support. We continually invest in our testing, capability, and state-of-the art technology to help you reduce risk, improve food safety and quality. For furthermore information, please visit our website: www.sgs.com/foodsafety.
For enquiries, please contact:
Shawn Yan
Health & Nutrition
SGS-CSTC Standards Technical Services(Shanghai) Co., Ltd.
5/F, 3rd Building No. 889, Yishan Road, Xuhui District, Shanghai, China
Phone: +86 (0)21 60645124




.webp?width=1644&height=1254&name=Food%20Safety%20Dashboard%201%20(1).webp)
