In recent years, an alarming discovery has been made regarding heavy metal contamination in the global food supply, specifically concerning toxic elements such as arsenic, lead, and cadmium. The U.S. Food and Drug Administration (FDA) has issued Import Alert 99-42, a critical directive to its field personnel, highlighting the severity of the issue and the need for heightened scrutiny.
The Global Threat
Toxic elements like arsenic, lead, and cadmium have been identified in certain foods, raising significant concerns about potential health risks. The FDA acknowledges the serious implications of these contaminants, especially for vulnerable populations such as infants and children. As a response, the agency actively monitors global food supplies, employing a systematic approach to mitigate the risks posed by these toxic elements.
FDA Action Levels and Proposals
The FDA has established action levels for toxic elements in specific foods, exemplified by limits set for arsenic in infant rice cereal and proposed levels for other items like fruit juices, including apple juice. The agency's website provides detailed information on these action levels, emphasizing a commitment to safeguarding public health. In cases where explicit limits haven't been defined, the FDA evaluates contaminants on a case-by-case basis, considering factors such as the type and amount of the toxic element and the population segment most at risk.
The "Closer to Zero" Action Plan
On April 8, 2021, the FDA introduced the "Closer to Zero" action plan, outlining strategies to minimize exposure to toxic elements in foods consumed by babies and young children. The plan reflects the agency's dedication to reducing these contaminants to as low as possible, given the heightened vulnerability of infants and young children due to their smaller body sizes and metabolism. The full action plan details can be accessed on the FDA's website.
Import Alert 99-42: A Critical Directive
Import Alert 99-42 serves as a vital tool in the FDA's arsenal to combat heavy metal contamination. This directive covers specific firms that have distributed foods containing levels of toxic elements that may endanger human health. The alert enables the detention without physical examination of such products, emphasizing the agency's commitment to ensuring the safety of the food supply.
Guidance and Detention Process
The FDA provides guidance for its divisions to detain shipments of identified products from the firms listed in the Red List of Import Alert 99-42. Recommendations for additions to the Red List are evaluated on a case-by-case basis by the Division of Import Operations and the FDA's Center for Food Safety and Applied Nutrition.
Securing Release and Removal from Detention
To release a shipment subject to detention without physical examination, the responsible party must provide evidence demonstrating that the product does not contain injurious levels of toxic elements. Private laboratory analysis is one method of providing such evidence. Firms may also be removed from detention by demonstrating resolution of the conditions that led to the violation.
SGS Digicomply Intelligence: Impact of FDA Import Alert 99-42 on Incident Numbers
As observed in the chart depicting incident numbers of Heavy Metal Contamination, including Arsenic, Lead, Cadmium from 2016 to 2023, a notable increase of approximately 1211% is evident. What accounts for this rise, and should we be concerned about further escalation? Let's delve into the details.
Import Alert 99-42 was initially published in 2021, with subsequent substantial modifications implemented in 2022 and 2023. In 2022, incident numbers surged, marking a pivotal point that might be associated with the stringent FDA regulations (Import Alert 99-42) enforced in the preceding year, 2021. It is plausible that many companies found themselves unprepared to meet the heightened standards. However, by 2023, a shift in the trend becomes apparent, reflecting a decline. This suggests that companies have potentially adapted more rigorously to comply with the regulations, signifying a positive response to the FDA's measures.
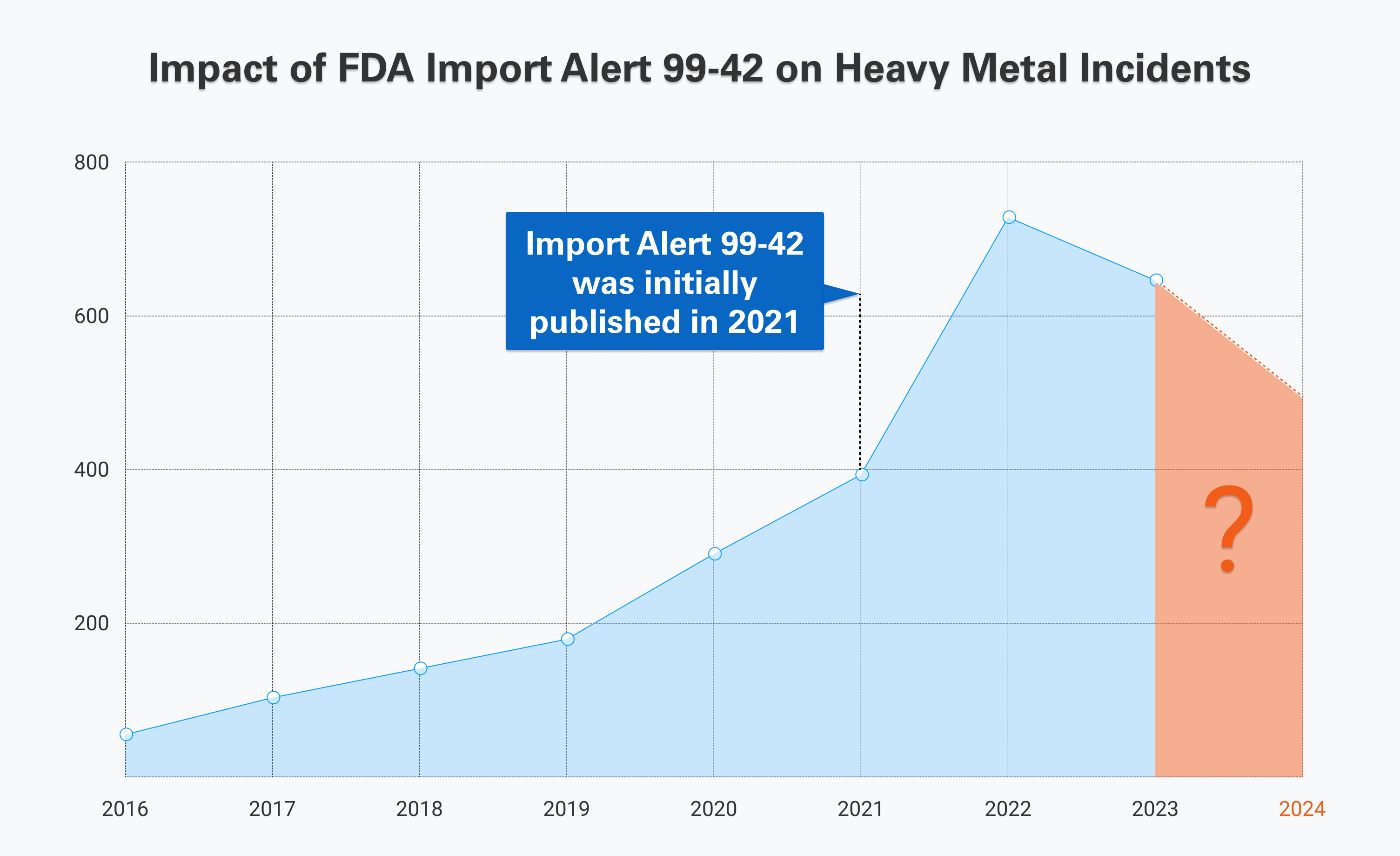
We anticipate that companies will persist in their adaptation efforts, leading to further reduction in incident numbers. However, it's essential to acknowledge that the future trend is contingent on potential changes that the FDA might introduce to Import Alert 99-42. It is crucial to remain vigilant and stay informed. SGS Digicomply serves as an invaluable resource for monitoring and responding to emerging developments in the realm of Heavy Metal Contamination, especially regarding arsenic, lead, and cadmium. Stay tuned to SGS Digicomply for ongoing insights and updates in this evolving landscape. Explore SGS Digicomply platform now.





.webp?width=1644&height=1254&name=Food%20Safety%20Dashboard%201%20(1).webp)
