Brominated Vegetable Oil (BVO) has been a topic of discussion and concern in the food industry, prompting the Food and Drug Administration (FDA) to consider its classification as a food additive. In this comprehensive exploration, we delve into the intricacies of BVO, its uses, potential health implications, and the reasons behind the FDA's proposal.
WHAT is Brominated Vegetable Oil
Brominated Vegetable Oil is a complex compound derived from vegetable oil, typically soybean or corn oil. Its primary function in the food and beverage industry is as an emulsifier, helping to distribute flavors evenly and prevent separation of ingredients. Additionally, BVO is employed to enhance the stability of citrus-flavored beverages, where it acts as a clouding agent.
Composition and Chemistry
To comprehend the FDA's stance on BVO, it's crucial to examine its composition. BVO contains bromine, an element belonging to the halogen group. The presence of bromine allows BVO to serve as a flame retardant, a characteristic that has raised concerns regarding its safety in food products.
Health Concerns
Several studies have suggested potential health risks associated with the consumption of products containing BVO. Bromine, when ingested in excessive amounts, may accumulate in the body and lead to adverse effects. Concerns include links to neurological symptoms, thyroid dysfunction, and reproductive issues.
International Variances in Regulation
The use of BVO varies globally, with some countries banning its use as a food additive due to health concerns. In the United States, the FDA has permitted its use within certain limits, but the agency is now reevaluating its classification in response to emerging research and consumer apprehensions.
REGULATORY BACKGROUND
BVO's history as a food ingredient dates back to the 1920s. In the late 1950s and early 1960s, the FDA considered BVO to be generally recognized as safe (GRAS) and placed it on the GRAS list. However, concerns about its safe use led to its removal from the GRAS list in the late 1960s. The FDA, recognizing the lack of sufficient data to restrict its use, limited BVO as a flavoring oil stabilizer in fruit-flavored beverages, and began regulating it as a food additive.
The FDA concluded in 1970 that the use of BVO in food was not GRAS due to toxicity concerns at the time. Subsequent safety studies, especially related to heart health, were conducted, and in 1970, the FDA began regulating BVO as a food additive with limitations on its use. Over the years, the FDA continued to evaluate new information on the possible health effects of BVO.
RECENT SCIENTIFIC STUDIES
Between 2016 and 2020, the FDA published improved methods for measuring BVO in commercial soft drinks and fats in vegetable oil. These advancements enabled the development of methods used in later animal studies, providing more accurate assessments of BVO levels in tissues.
A crucial study published on May 16, 2022, in the journal Food and Chemical Toxicology evaluated potential health effects related to BVO consumption in rodents. The study indicated that oral exposure to BVO is associated with increased tissue levels of bromine and identified the thyroid as a potential target organ for negative health effects, especially at high levels of exposure.
FDA'S PROPOSED RULE
Culminating from the wealth of accumulated data, on November 2, 2023, the FDA issued a proposed rule that, if finalized, would revoke the regulation allowing the use of BVO in food. The decision is rooted in a reassessment of animal and human data, including information from recent FDA-led studies, which no longer provide a basis to conclude that the use of BVO in food is safe.
The debate surrounding Brominated Vegetable Oil emphasizes the need for continuous scrutiny of food additives and their potential impacts on health. As the FDA navigates the complexities of BVO, ongoing research, consumer awareness, and regulatory actions will play pivotal roles in shaping the future of food safety and transparency.
SGS Digicomply: PROACTIVE REGULATORY CHANGE MANAGEMENT
As the discussion on Brominated Vegetable Oil (BVO) unfolds, it becomes imperative to stay vigilant and proactive. Monitoring all mentions, regulatory proposals, and changes is crucial for instant and effective risk prevention. Notably, regulatory documents and additional information from this article can be easily accessed through the SGS Digicomply Regulatory Intelligence Hub.
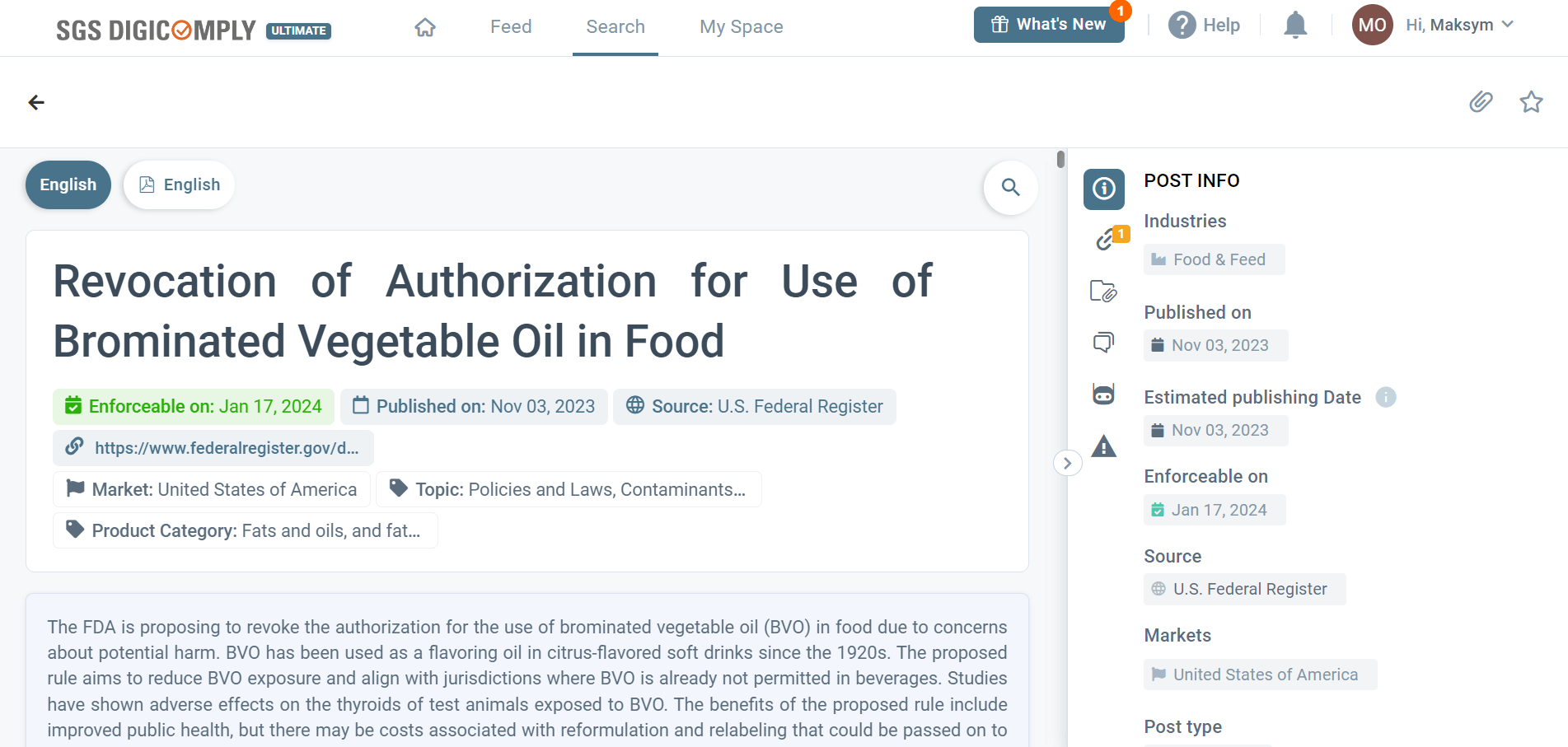
Leveraging cutting-edge AI technology, SGS Digicomply consistently monitors and analyzes a vast array of data sources, providing valuable and up-to-date insights on triggers for regulatory changes. This proactive approach is ideal for a comprehensive analysis of the dynamic interplay between scientific research, regulatory decisions, and the intricate landscape of regulatory change management in the modern era.
Feel free to get in touch now to learn about implementing the Regulatory Intelligence Hub for your company.
COnclusion:
In conclusion, the evolving narrative around Brominated Vegetable Oil underscores the dynamic interplay between scientific research, regulatory decisions, and public health considerations. The proposed rule invites further dialogue and reflection on the delicate balance between innovation in the food industry and ensuring the well-being of consumers. As the regulatory process unfolds, stakeholders across the food supply chain will closely monitor developments in this critical aspect of food safety.





.webp?width=1644&height=1254&name=Food%20Safety%20Dashboard%201%20(1).webp)
