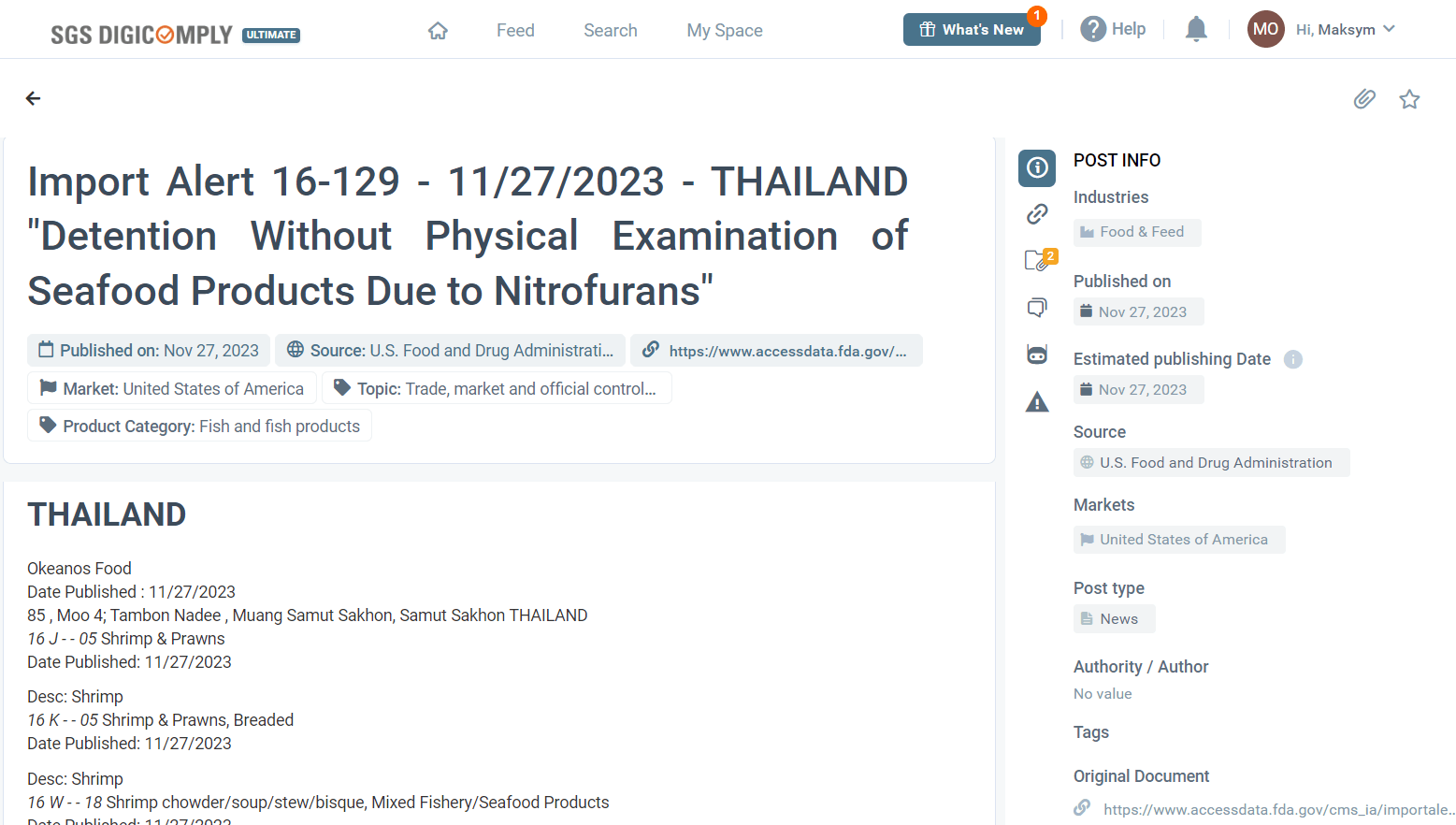
In a significant development for the food safety community, nitrofurans and nitrofurazone have been detected in seafood products, leading to regulatory actions. This situation, identified through diligent monitoring by regulatory authorities, emphasizes the potential health risks to consumers and highlights the necessity of stringent food safety protocols. As a direct response, the FDA issued Import Alert 16-129 on 11/27/2023 - THAILAND "Detention Without Physical Examination of Seafood Products Due to Nitrofurans". SGS Digicomply Early Warning identified this notification, and we are eager to share this important update with you in this Food Safety Snapshot.
Feel free to get in touch now to learn about implementing the Food Safety Intelligence Hub for your company.
WHAT ARE NITROFURANS: RISKS AND IMPLICATIONS
Nitrofurans are synthetic, broad-spectrum antibiotics that have been used to combat bacterial infections in both humans and animals. Due to their effective action against a wide array of gram-positive and gram-negative bacteria, they were widely utilized in veterinary medicine, including aquaculture. However, the stability of nitrofuran residues and their potential health risks upon consumption have led to significant regulatory scrutiny and restrictions. These compounds, when ingested, can bind to cellular macromolecules, leading to concerns over their carcinogenic and genotoxic potential. Consequently, the use of nitrofurans in food-producing animals has been prohibited in many jurisdictions, including the United States, due to the risks they pose to human health.
NITROFURANS Identified in Seafoods, Leading to Increased Scrutiny
The identification of nitrofurans and their metabolite nitrofurazone in seafood products marks a critical point in food safety oversight, indicating a potential lapse in food safety protocols. This finding is particularly alarming due to the health risks it poses to consumers and the implications for the seafood supply chain's integrity. The presence of these contaminants underscores the vital need for continuous monitoring and strict enforcement of food safety standards to ensure public health protection.
SGS Digicomply Early Warning: FDA's Import Alert 16-129 Response
The FDA's response to the detection of nitrofurans in seafood products has been swift and decisive, culminating in the issuance of Import Alert 16-129. This alert specifically targets seafood products from Thailand, mandating "Detention Without Physical Examination" (DWPE) of products suspected to contain nitrofurans. The alert outlines the FDA's guidance for field personnel, empowering them to detain products from certain firms without the need for physical examination. This measure is aimed at preventing the entry of contaminated seafood into the United States, thereby safeguarding consumer health.
Key Details of the Import Alert:
- Scope and Products Affected: The alert applies to a broad range of seafood products, including but not limited to shrimp and prawns (both unprocessed and breaded), shrimp chowder/soup/stew/bisque, stuffed pasta with shrimp, shellfish dumplings, and various mixed fishery/seafood products.
- Firms and Locations: One of the specifically mentioned firms is Okeanos Food, located in Samut Sakhon, Thailand, underscoring the geographical focus of the alert.
- Guidance for Manufacturers: Manufacturers whose products are targeted by this alert are urged to ensure that their products are free from nitrofurans before exporting to the United States. Compliance with FDA regulations and proactive engagement in safety and quality testing are essential steps in mitigating the risk of detention and ensuring access to the U.S. market.
- Analysis and Enforcement: The FDA has detailed the procedures for collecting and analyzing samples of the implicated products, emphasizing the use of validated methods to identify nitrofuran residues. Products testing positive for these substances are to be refused entry under specific charges related to the safety and regulation of new animal drugs and food additives.
Conclusion
The detection of nitrofurans in seafood products and the subsequent issuance of Import Alert 16-129 by the FDA mark a significant event in the realm of food safety. This development not only highlights the challenges of maintaining a safe and secure global food supply chain but also the importance of rigorous regulatory oversight and industry compliance. As regulatory bodies like the FDA continue to adapt and respond to emerging threats to food safety, the role of early warning systems like SGS Digicomply becomes ever more critical. These systems serve as a vital component in the early detection and management of food safety risks, protecting consumers and ensuring the integrity of the food supply.





.webp?width=1644&height=1254&name=Food%20Safety%20Dashboard%201%20(1).webp)
