Ready-to-Eat Foods Lead Food Recalls in 2025: Understanding the Growing Risk
Ready-to-eat foods have emerged as the dominant category for food recalls in 2025, representing a...
Discover the latest insights from SGS DIGICOMPLY

Ready-to-eat foods have emerged as the dominant category for food recalls in 2025, representing a...

Ready-to-eat foods have emerged as the dominant category for food recalls in 2025, representing a...

From supermarket shopping to take-away meals, consumers today encounter an abundance of disposable...
Read more
The global gluten-free market has grown rapidly over the past decade, driven by increased diagnosis...
Read more
The European Union has taken a decisive step to protect consumer health by adopting a sweeping ban...
Read more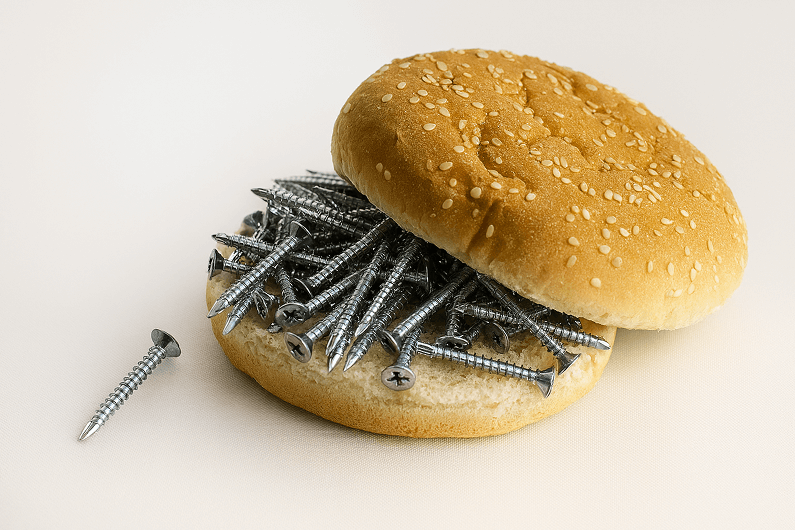
Foreign object contamination — from plastic shards to metal fragments — is no longer rare. Over...

If you work with plastic materials intended to come into contact with food in the EU, it’s time to...
Read more
Salt has shaped cuisines, preserved food for centuries, and made snack foods irresistible. But...
Read more
Food shoppers around the world are beginning to find something new in their carts – foods made with...
Read more
In 2025, Malaysia is making a significant shift in food safety policy. The country’s new Food...
Read more
Energy drinks – packed with caffeine and other stimulants – have surged in popularity worldwide....
Read more
For years, bottled mineral water has been the go-to choice for health-conscious consumers — a...

The reintroduction of widespread tariffs by the United States is having a profound—though often...
Read more