Microalgae-based biopolymers have been “on the horizon” for years. Most sustainability...
GB 2762-2025: China's Updated Food Contaminant Limits
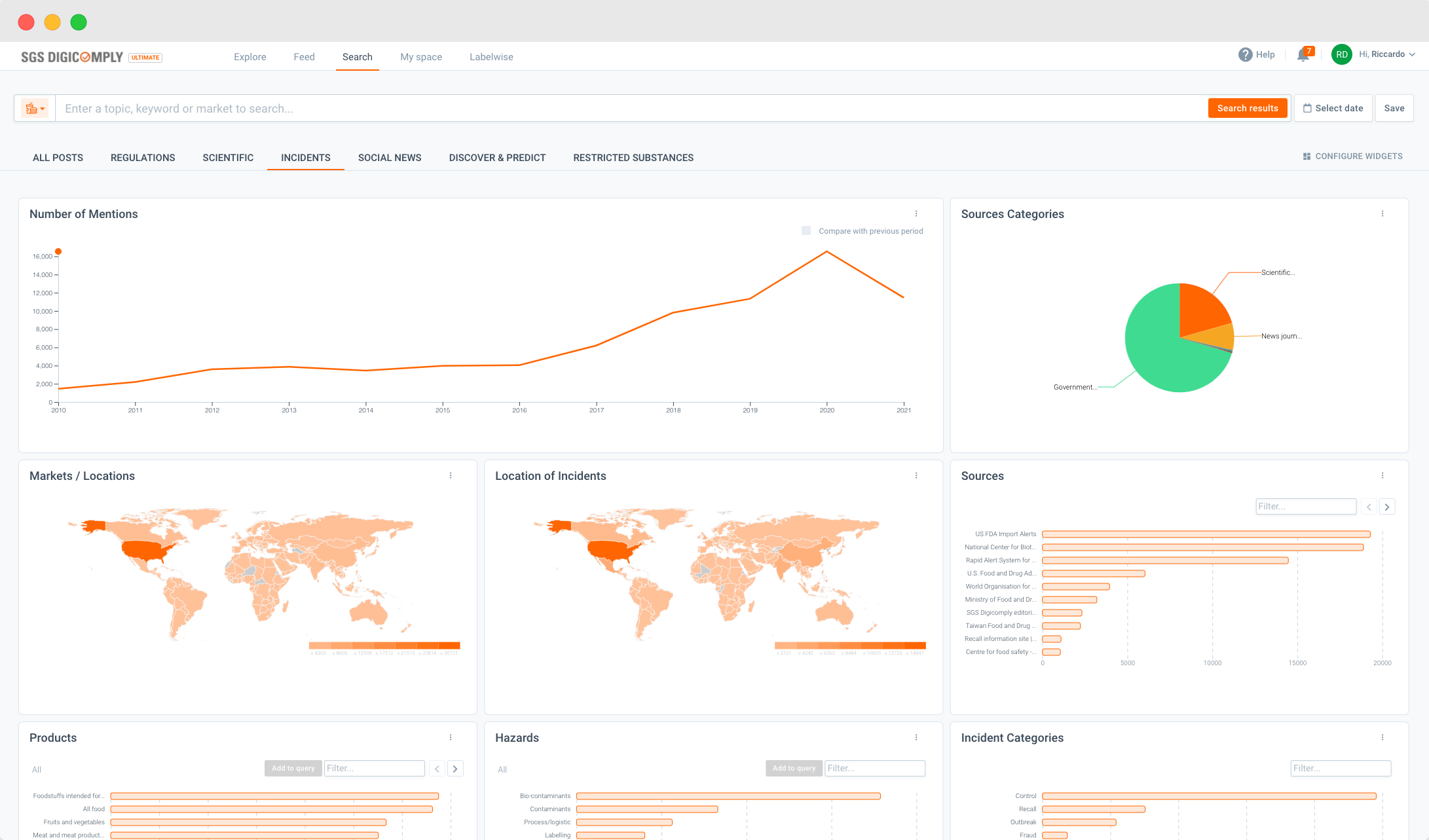
China released GB 2762-2025, the updated National Food Safety Standard for contaminant limits in...
Read Article